

#Blueprint medicines stock drivers#
"In 2023, Blueprint Medicines has a diversity of fundamental value drivers that position us well on the path to achieve our 2027 Blueprint for Precision at Scale and translate the promise of precision medicine into reality for thousands of patients globally."įourth Quarter 2022 Highlights and Recent Progress In addition to our commercial execution, we advanced additional clinical development programs and continued to bolster our financial strength through our strategic financing and business development," said Kate Haviland, Chief Executive Officer of Blueprint Medicines. Today, we are at the precipice of a pivotal moment in Blueprint's history as we near a potential FDA approval of AYVAKIT for a much broader population of patients with indolent SM, which will enable us to address the significant medical needs of SM patients and create a critical inflection point in our growing revenue stream. "In 2022, AYVAKIT became the standard of care for patients with advanced systemic mastocytosis in the U.S., and we continued to solidify our global leadership in mast cell disorders.
#Blueprint medicines stock update#
16, 2023 /PRNewswire/ - Blueprint Medicines Corporation (NASDAQ: BPMC) today reported financial results and provided a business update for the fourth quarter and full year ended December 31, 2022.
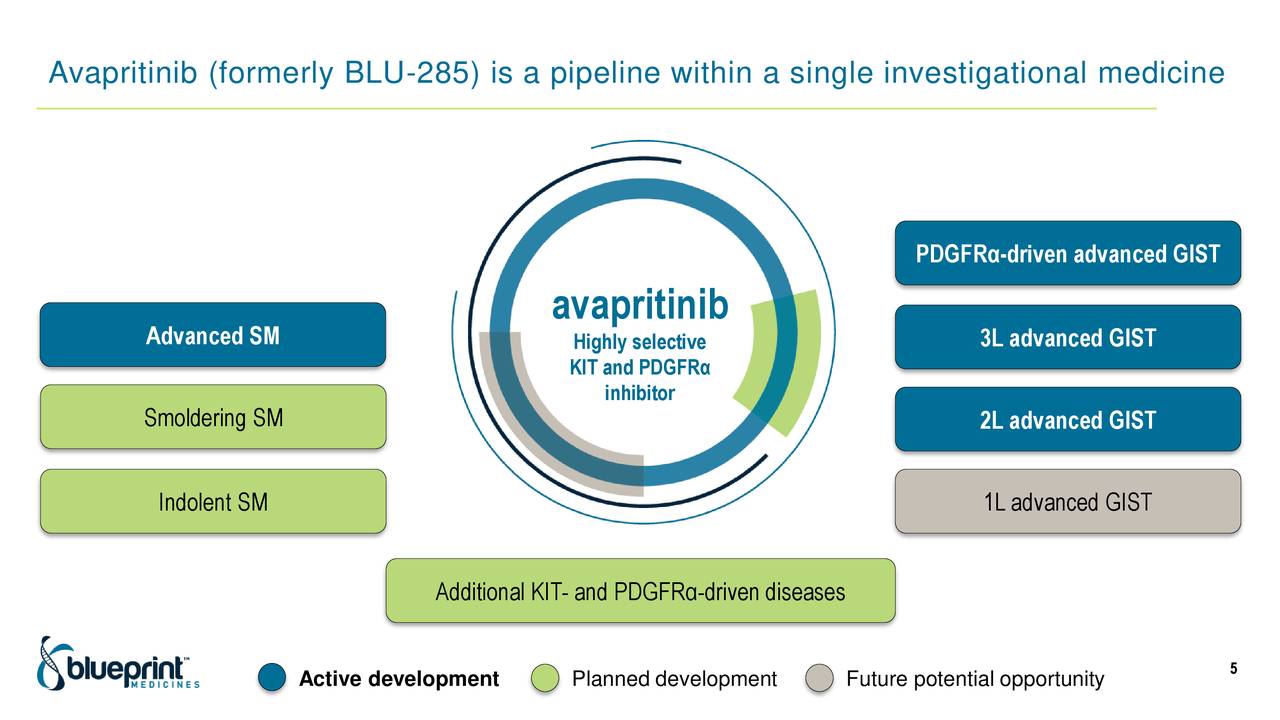
Positive data from PIONEER trial of AYVAKIT in indolent systemic mastocytosis to be presented at the AAAAI Annual Meeting -ĬAMBRIDGE, Mass., Feb. Continued progress toward regulatory approvals of AYVAKIT/AYVAKYT for indolent systemic mastocytosis, with FDA granting priority review of a supplemental new drug application and EMA validating a type II variation marketing authorization application. Achieved $204.0 million in total revenues in 2022, including $111.0 million in AYVAKIT®/AYVAKYT® (avapritinib) net product revenues.


 0 kommentar(er)
0 kommentar(er)
